Substance Abuse in Children Ages 11-13 Peer Reviewed
Interventions for Substance Use Disorders in Adolescents: A Systematic Review
Research Protocol Oct 29, 2018

I. Background and Objectives for the Systematic Review
In 2015, in the Us, an estimated 1.3 1000000 adolescents, aged 12 to 17, and 5.4 million young adults, aged eighteen to 25, met diagnostic criteria for having a substance use disorder (SUD); the vast bulk were untreated.i Adolescents with SUD are at take a chance of experiencing a cascade of far-reaching adverse outcomes that often persist into adulthood, including sexually transmitted infections,2 unintended pregnancy,3 criminal involvement,4 school truancy,v psychiatric disorders,vi and physical health problems.7 Boyish substance use is associated with the leading causes of death in this age cohort: suicide, unintentional injury, and violence.8,9
Prescription and over-the-counter medications are the most commonly misused substances, after alcohol, marijuana, and tobacco, among 12th graders;ten with one percent of youth betwixt the ages of 12 and 17 reporting electric current opioid misuse.eleven Youth who use opioids are more likely to use other substances.x Among youth under 21 who initiate heroin use, 80 pct misused prescription and/or over-the-counter medication before the age of eighteen.12 National concerns about opioid misuse, encompassing nonmedical apply of prescription opioid-based medications (east.g., morphine, fentanyl) and the use of illegal opiates (e.g., heroin), have brought heightened attention to the significant gamble of drug overdose death in adolescents.13
The pervasive negative consequences associated with untreated or ineffectively treated adolescent substance use (SU), and the loftier lethality of opioid misuse in particular, underscore the importance of treating substance use in adolescents.
In 2005, the American Academy of Child and Boyish Psychiatrists (AACAP) created a Practice Parameter (PP) for the Cess and Handling of Children and Adolescents with SUDs. The 2005 Practise Parameters fabricated eight recommendations pertaining to handling. For behavioral treatments, AACAP concluded that family unit therapy models "have the most supporting evidence" and "individual approaches such as cognitive-behavioral therapy, both lone and with motivational enhancement therapy, accept been shown to be efficacious." AACAP recommended that "medication can be used when indicated," noting that this recommendation was "not based on empirical research in adolescents but rather on research and feel with adults."14 The AACAP also recommended that psychiatrists consider co-occurring mental health disorders, since the bulk of adolescents with substance use problems present with a co-occurring mental health diagnosis. Recommendations made in the 2005 PP were limited past a relative lack of rigorous trials at the fourth dimension.
Since the publication of the initial PP, in that location has been a proliferation of boyish substance use handling trials, many of which have employed more rigorous designs, larger samples, random assignment, direct comparisons of 2 or more than active treatments, improved measures of substance use and other variables, manual-guided interventions, and longer-term consequence assessments. Reviews of the adolescent substance use literature take typically focused only on behavioral treatments,xv-17 pharmacologic handling of a specific SUD,18 or on a specific handling model (e.g., motivational interviewingxix and screening, brief interventions, and referral to handling).twenty In 2014, a guide developed by the National Institute of Drug Abuse (NIDA), identified multiple approaches to treating adolescent SUDs, which were divided into behavioral approaches, family-based approaches, habit medicine, and recovery support services, but this report did not synthesize evidence on comparative effectiveness.21 The American Academy of Pediatrics (AAP) Committee on Substance Apply and Prevention recommended consideration of pharmacotherapy for adolescent and immature adult patients with astringent opioid use disorders or co-occurring booze use disorders.22 Thus, there is a meaning need for a rigorous and comprehensive synthesis of the adolescent substance utilize treatment literature that addresses both pharmacological and psychological treatments.
The planned systematic review (SR) will inform a Clinical Update and Clinical Practice Guideline to update the 2005 AACAP PP for the Assessment and Treatment of Children and Adolescents with SUDs. Given the high co-occurrence of substance use and other mental illnesses, and the increased focus on integrated treatment, at that place is significant need and opportunity to engage and educate psychiatrists likewise as main care physicians.23
The overarching goal of the review is to evaluate the available show for the treatment effects (and comparative effects) of available behavioral and pharmacologic interventions. In addition, the review will evaluate treatment effects beyond population subgroups and place evidence (or gaps in evidence) regarding the key ingredients of successful interventions for problematic substance use in adolescents.
Ii. The Central Questions
The post-obit are the Key Questions (KQs) to be addressed by this systematic review:
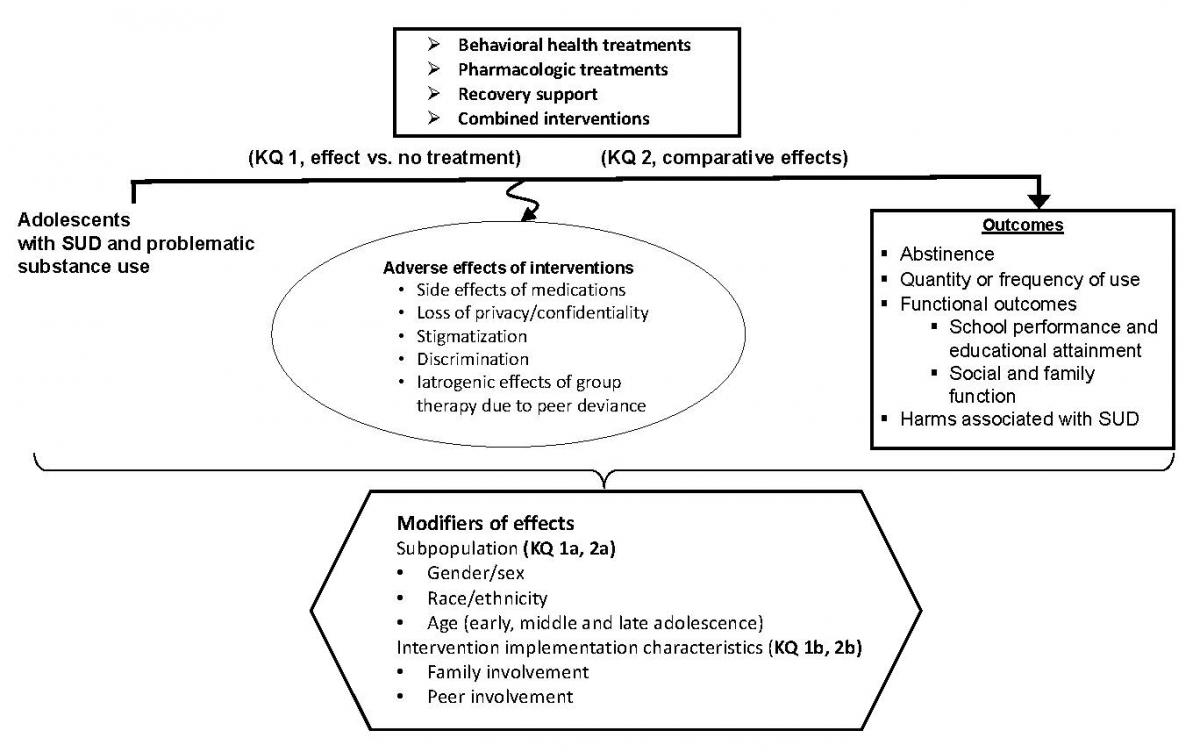
KQ 1: What are the effects of behavioral, pharmacologic, and combined interventions compared with placebo or no active treatment for substance utilise disorders and problematic substance use[i] in adolescents to reach forbearance, reduce quantity and frequency of use, amend functional outcomes, and reduce substance-related harms?
- How do benefits and adverse outcomes of interventions vary by subpopulations?[2]
- How do benefits and adverse outcomes of interventions vary by intervention characteristics?[3]
KQ 2: What are the comparative effects of active interventions for substance use disorders and problematic substance use1 in adolescents to attain forbearance, reduce quantity and frequency of utilize, ameliorate functional outcomes, and reduce harms?
- How practice comparative benefits and adverse outcomes of interventions vary by subpopulations?2
- How do comparative benefits and adverse outcomes of interventions vary by intervention characteristics?3
III. Study Eligibility Criteria
Population (all KQs)
- Age: Adolescents (12–20 years inclusive)
- Exclude if > 20 percent of study sample (or identifiable subgroup) is <12 or >twenty years, combined
- SUD or problematic apply of:
- Alcohol
- Exclude primary studies of treatment of alcohol utilise disorder/problematic alcohol employ in the higher setting (nosotros will include existing systematic reviews)
- Cannabis
- Opioids
- Nonmedical prescription drug use (codeine, hydrocodone, oxycodone)
- Illicit (e.1000., heroin, illicit synthetics)
- Sedatives, hypnotics, or anxiolytics (e.grand., benzodiazepines, carbamates, barbiturates, methaqualone)
- Stimulants
- Nonmedical prescription drug use (e.thousand., methylphenidate)
- Illicit (eastward.thousand., cocaine, methamphetamine)
- Inhalants
- Hallucinogens (e.g., phencyclidine, ketamine, MDMA, LSD)
- Unspecified or polysubstance utilise
- Exclude if predominately tobacco/nicotine use
- Exclude tobacco/nicotine apply disorder or problematic tobacco/nicotine use
- Exclude limited (or experimental) substance use that has not been deemed to be at least "problematic"
- Alcohol
- Subpopulations of interest (non necessary for eligibility)
- Psychiatric comorbidities
- Attention deficit hyperactivity disorder (ADHD), depression, other internalizing and externalizing disorders.
- Age
- Early adolescence (12–fourteen years)
- Eye adolescence (15–17 years)
- Late adolescence (xviii–20 years)
- Sex and gender
- Male vs. female person
- Gender identity (cis vs. transgender)
- Sexual orientation
- Racial/ethnic minority
- Socioeconomic status and related characteristics (east.g., homelessness, poverty)
- Pregnant, postpartum, and parenting adolescents
- Demographic/family characteristics
- Demographics
- Family and community dynamics (i.due east. substance using family unit member)
- Involvement with kid protection services.
- Psychiatric comorbidities
Interventions
- Behavioral wellness treatments (major intervention models are indicated past arrowhead bullets, in assuming)
- Family unit Therapies
- Family behavioral therapy (FBT)
- Family unit systems therapy (FST)
- Brief strategic family therapy (BSFT)
- Functional family therapy (FFT)
- Ecological family therapy
- Multidimensional family therapy (MDFT)
- Ecologically based family therapy (EBFT)
- Family systems network (FSN)
- Educational family therapy
- Multi-systemic therapy (MST)
- Cerebral behavioral therapy (CBT)
- Adolescent community reinforcement arroyo (ACRA)
- Dialectical beliefs therapy
- Cognitive therapy
- Contingency management
- Motivational interviewing/ Motivation enhancement therapy
- Multi-component interventions consisting of two or more models (e.one thousand., MST + CBT; FFT + CBT)
- Psychoeducation
- Treatment as usual (does not meet criteria for any of the above categories)
- Integrated interventions for substance use and a co-occurring disorder
- Other
- Culturally sensitive interventions
- Recovery support
- 12-step programs
- Peer-based and/or peer supports
- Believing standing care (ACC)
- Exclude primary (universal) and secondary preventive interventions.
- Exclude interventions used in population that practise non aim to reduce substance employ (e.g., needle exchange).
- Family unit Therapies
- Pharmacologic interventions
- Exclude medications being used to treat overdose (due east.thou., naloxone)
- Exclude pharmacologic direction of astute withdrawal symptoms
- Medications to reduce and/or eliminate substance use and to prevent relapse
(Run into Appendix B for details of FDA approvals)- Booze
- Gabapentin
- Naltrexone
- Acamprosate
- Disulfiram
- Topiramate
- Ondansetron
- Cannabis
- N-acetylcysteine (NAC)
- Opioids
- Methadone
- Buprenorphine
- Buprenorphine/Naloxone
- Naltrexone
- Booze
- Medications to treat co-occurring psychiatric disorders in patients in patients with concurrent problematic substance employ or SUD.
Comparators
- KQ 1
- No active treatment
- Wait list
- Placebo (for medications)
- Usual care (if not a clearly defined behavioral intervention)
- No active treatment
- KQ 2
- Active interventions (we will evaluate other comparisons if the bear witness allows)
- Pharmacologic plus behavioral vs. behavioral or pharmacologic alone
- Between major behavioral intervention models (due east.g. family unit therapy, cognitive behavioral therapy)
- Multicomponent interventions vs. single behavioral intervention model
- Active interventions (we will evaluate other comparisons if the bear witness allows)
Outcomes
- Abstinence
- Urine drug test results (from substance identified on access to treatment, forbearance from all substances, duration of abstinence)
- Quantity, frequency, or severity of employ (of chief substance identified on entry to treatment and other substances)
- Days of use/abstinence over specified time period
- Quantity of utilise over specified fourth dimension menstruation
- Substance-related bug/symptom count scales
- Functional outcomes
- School performance and educational attainment
- Attendance
- Grades/academic operation
- Graduation rates
- Entering higher education (including trade schools)
- Social relationships
- Family functioning
- Peer relationships
- School performance and educational attainment
- Harmful consequences associated with SUD
- Mental health outcomes
- Suicidal ideation and behavior
- Physical wellness outcomes
- Bloodshed
- All-crusade
- Drug-related, including fatal overdose
- Morbidity
- Injuries (non-fatal)
- Infections
- HIV
- Hepatitis C
- Other sexually transmitted infections
- Bloodshed
- Legal outcomes
- Arrests
- Boozer or impaired driving
- Contact with juvenile justice system
- Mental health outcomes
- Adverse furnishings of intervention(south)
- Side effects of pharmacologic interventions
- Loss of privacy/confidentiality
- Stigmatization/discrimination
- Iatrogenic effects of group therapy due to peer deviance
- Other reported agin effects ascribed to interventions
Report Designs and Data Sources
- Published, peer reviewed manufactures and data from clinicaltrials.gov
- Randomized controlled trials (including cross-over trials)
- N ≥10 participants per report group
- Large nonrandomized comparative studies with longitudinal follow-up
- N ≥ 100 participants per report grouping
- Must report multiple regression, other adjustment, matching, propensity scoring, or other method to account for misreckoning.
- Single arm pharmacologic studies with at least 200 participants and longitudinal follow-up (to identify side-effects of medications)
- We will summarize information from existing systematic reviews specific to treatment of booze SUD on college campuses
- SR eligible if inclusion criteria for private studies consequent with our PICODT criteria for individual studies.
- Randomized controlled trials (including cross-over trials)
- Exclusions
- Instance-command studies
- Cross-sectional studies
- Single-arm studies of behavioral interventions
- Conference abstracts letters, and other non-peer reviewed reports
Timing
- Whatever elapsing of treatment
- Duration of follow-upward of at least a month (but must be longitudinal with separation in time between intervention and outcomes)
Setting
- Any setting, including (only not express to) primary care, school, outpatient, emergency department, in-patient, intensive outpatient, partial hospitalization, intensive inpatient/residential, juvenile justice
- Exclude: laboratory-based assessments.
IV. Analytic Framework for the Key Questions
V. Methods
Criteria for Inclusion/Exclusion of Studies in the Review
Please refer to Section II, The Cardinal Questions, where the Eligibility Criteria are listed.
Searching for the Show
Literature Search Strategies for Identification of Relevant Studies to Answer the Key Questions
Nosotros will conduct literature searches in MEDLINE, the Cochrane Key Trials Registry, EMBASE, CINAHL, and PsycINFO databases (from inception) to identify master studies meeting our criteria. A separate search for SRs of interventions for alcohol are disorders/problematic alcohol apply in the college setting will be conducted in MEDLINE, Cochrane Database of Systematic Reviews, and Epistemonikos. Nosotros anticipate using the search strategy in Appendix A, which is designed for the PubMed interface of MEDLINE, adapted as needed for each database. The search strategy will be peer reviewed by an independent, experienced information specialist/librarian. We will ask the Technical Expert Panel (TEP) to provide citations of potentially relevant articles. Additionally, we volition peruse the reference lists of published clinical practice guidelines, and relevant systematic reviews (as identified in MEDLINE, the Cochrane Database of Systematic Reviews, and Epistemonikos) for eligible studies. For evaluation of the handling of booze utilise disorders/problematic alcohol apply in the college setting, we volition summarize existing systematic reviews only, as this literature is vast and has been extensively reviewed. Nosotros volition search ClinicalTrials.gov to identify unpublished studies and studies that are ongoing. We will also search the FDA websites for pharmacologic trials.
Peer and public review will provide an boosted opportunity for the TEP and other experts in the field to ensure that no key publications have been missed. We will update the search upon submission of the typhoon written report for peer and public review.
Finally, a Supplemental Evidence and Data for Systematic review (SEADS) portal and Federal Register Notice volition be posted for this review.
Screening Studies for Eligibility
For citation screening, we will initially conduct a series of airplane pilot training sessions to attain a satisfactory level of agreement amongst researchers regarding the nuances of the eligibility criteria for title and abstract screening. Because abstracts sometimes practice not mention all outcomes that are reported in the full-text, we volition not exclude titles and abstracts based on outcomes. We volition carry all abstract screening using the open-source, online software Abstrackr. Nosotros volition use the predictive algorithm capabilities of Abstrackr to aid with screening. We will begin with double, independent screening of abstracts. Conflicts will be resolved during full-group meetings. Using the labels (have, reject) given to screened abstracts, Abstrackr will determine a prediction value for all remaining unscreened citations and sort these such that the most-likelyhoped-for-accepted abstracts are screened first. Based on empirical enquiry on Abstrackr (that is soon to be submitted for publication), when all remaining unscreened abstracts have a prediction value <0.40 (on a scale of 0 to 1), we volition switch to single screening of remaining abstracts. The empirical inquiry suggests that at this threshold, all remaining abstracts will be rejected. Typically, this threshold is reached when about half the abstracts have been screened.
We will obtain the full-texts of all citations that are screened in during abstract screening. The reference lists from systematic reviews will be reviewed for the presence of additional primary studies. will be We will evaluate these manufactures using an Evidence Map structure in which we will assemble bones information on each article (e.g., study design, sample size, confirmation of SUD/problematic use, age data, intervention(s), confirmation of outcomes of interest). Articles derived from the same studies (multiple publications, secondary analyses) will be grouped. This prove map process volition help to determine final eligibility status for each study.
Information Extraction
Each written report with multiple publications or secondary analyses will be extracted together (as one written report). I methodologist will excerpt data for each study. The extraction volition be verified past at least one other experienced methodologist and discrepancies will be discussed between them, every bit needed.
Information will be extracted into customized forms in the Systematic Review Data Repository (SRDR) online system and Excel spreadsheets designed to capture all elements relevant to the KQs. Upon completion of the review, the Excel spreadsheets will be uploaded into SRDR and the SRDR database will be published (made accessible to the public, with capacity to read, download, and comment on information).
The basic elements and pattern of these forms volition be the similar to those we take used for other comparative effectiveness reviews and will include elements that address population characteristics; descriptions of the interventions and comparators; result definitions; effect modifiers; enrolled and analyzed sample sizes; study design features; funding source; results; and chance of bias.
We do not plan to contact study authors for additional data.
Cess of Risk of Bias
We volition assess the risk of bias (methodological quality) of each study based on predefined criteria. For RCTs, we will employ the Cochrane risk of bias tool24 assessing randomization method and adequacy, resource allotment darkening method and adequacy, utilise of intention-to-treat assay, and masking (blinding). For observational studies, we will apply relevant questions from the Newcastle Ottawa Scale.25 For SRs of interventions for alcohol utilize disorder or problematic alcohol utilize in the college setting, we will assess risk of bias using the AMSTAR 2 tool.26 Whatsoever quality issues pertinent to specific outcomes within a report volition be noted and practical to those outcomes. Whatever quality issues pertinent to specific outcomes within a written report will be noted and considered when determining the overall force of evidence for conclusions related to those outcomes.
Data Synthesis
Synthesis
We will summarize all included studies in narrative form and in summary tables containing the of import features of the study populations, design, interventions, outcomes, and results. Tables will include descriptions of the report design, sample size, intervention(s), followup elapsing, outcomes, and study quality.
Nosotros will analyze different study designs separately and, if appropriate, together. We will compare and contrast populations, exposures, and results across written report designs. Nosotros will examine any differences in findings betwixt randomized and nonrandomized studies. We will evaluate the gamble of bias factors every bit possible explanations for any heterogeneity.
We may conduct random furnishings model meta-analyses of comparative studies if at least 3 studies are sufficiently like in population, interventions, outcomes, and report design. Specific methods and metrics (summary measures) to be meta-analyzed volition depend on available, reported written report information, but we look to summarize odds ratios of chiselled outcomes and, if pertinent, standardized mean differences of cyberspace change of continuous outcomes (e.yard., quality of life scores). Statistical heterogeneity volition be explored qualitatively and, if appropriate data are available, we may as well behave meta-regression analyses to evaluate study, patient, and intervention features, (as listed in the KQs) and to evaluate dose-response. Nosotros will explore subgroup differences within (and perhaps across) studies based on the list of comparisons described in the KQs. Nosotros will explore the possibility of conducting a network meta-analysis of clinical outcomes to compare handling alternatives across studies. Nosotros will also explore the use of hierarchical (random intercept/random slope) meta-regression analyses to tease out the additive effect of each intervention attribute. Sensitivity analyses will examine robustness of results to culling prior distributions; attribute definitions, and non-additivity of intervention components. Every bit needed, we will use methods for the multivariate pairwise and multiple-treatment meta-analysis of correlated outcomes.
Grading the Strength of Prove for Major Comparisons and Outcomes
We volition grade the strength of the body of evidence as per the AHRQ methods guide on assessing the force of evidence.27,28
We expect that we volition have at least some information for a multitude of comparisons. Yet, it is impractical to provide strength of evidence assessments for all possible combinations of interventions and outcomes. We will assess the strength of testify for comparisons of major interventions (i.e., behavioral intervention methods, pharmacologic interventions, and combinations) to no treatment and to each other that take at least 3 comparative studies or at to the lowest degree 1000 participants in total. Comparisons with only i or two smaller studies (in full Northward<m) will likely take insufficient or very low strength of show because of imprecision; for such comparisons we do not programme to formally evaluate and present the forcefulness of prove. While this a priori threshold is capricious, it is consequent with the concept that for imprecise prove "any gauge of event is very uncertain," the definition of Very Low quality bear witness per Form.29 Based on further evaluation of the evidence base, nosotros may lower the minimum sample size from 1000 for when there are fewer than three similar studies, especially if there are large effects ( i.e., standardized mean difference ≥0.8; see next paragraph), especially for patient-centered outcomes.
To our noesis, in that location is no information on the minimal clinically importance differences for the outcomes we consider. We therefore define a priori cutoffs in the magnitude of the intervention consequence to categorize consequence magnitudes. For continuous outcomes nosotros will consider small, modest, and large effects to correspond to standardized mean differences smaller than 0.2, between 0.2 and 0.eight, and at to the lowest degree 0.8, respectively, in either direction. By this definition, modest furnishings represent to a change in the mean of the outcome that is more than extreme than the measurements in sixteen% of the population in the controls. Moderate furnishings correspond to mean changes that are more than extreme than the measurements in 16% to 58% of the controls, and large effects to changes that are more extreme than the measurements in 58% of the controls. For non-rare categorical outcomes (operationally defined as having prevalence >5%), nosotros volition consider small odds ratios that are betwixt 1 and 1.2 (or between i and 0.83, in the other direction), moderate odds ratios that are between i.2 and two.0 (or between 0.83 and 0.5), and large odds ratios >2 (or <0.5). For rare categorical outcomes (with observed prevalence <v%) we volition non make such judgments, unless the outcomes are disquisitional (namely, overall mortality, crusade-specific mortality, or suicide attempts).
These effect size magnitudes volition be used as a proxy of whether a deviation betwixt two treatments is likely to be clinically of import: For statistically significant deviation with indicate estimates that are pocket-size or large in magnitude, we will deem that, in terms of clinical importance, the favored intervention is favored "moderately" or "strongly," respectively. When the intervention effect is minor in magnitude and/or statistically nonsignificant, we will consider a difference every bit "clinically not important."
Conversely, we will estimate the evidence on "clinical equivalence" between ii interventions based on the 95 percent confidence intervals of the intervention effects. We operationally ascertain that there is strong evidence of clinical equivalence if the premises of the conviction interval exclude effects of at to the lowest degree moderate magnitude equivalence; and moderate evidence of clinical equivalence when the 95 percent conviction intervals exclude large effects, but not moderate effects, in either management.
For each evaluated comparison, we will assess the number of studies, their study designs, the written report limitations (i.e., risk of bias and overall methodological quality), the directness of the evidence to the KQs, the consistency of study results, the likelihood of reporting bias, in addition to the precision and magnitude of the result judge across studies. Based on these assessments, nosotros will assign a strength of prove rating equally being either high, moderate, low, or insufficient evidence to approximate an effect. The data sources, basic study characteristics, and each strength-of-evidence dimensional rating will exist summarized in a "Summary of Evidence Reviewed" table detailing our reasoning for arriving at the overall forcefulness of evidence rating.
Assessing Applicability
We will assess the applicability within and across studies with reference to adolescents in the populations of interest (i.e. blazon and severity of abuse, early vs. middle vs. late adolescent age grouping and setting), and whether interventions and comparators are used in current practice.
Six. References
- Lipari RN, Park-Lee E, Van Horn S. America's Need for and Receipt of Substance Employ Treatment in 2015. The CBHSQ Report. Rockville (MD); 2016:ane–7.
- Dembo R, Belenko S, Childs K, et al. Drug use and sexually transmitted diseases among female person and male arrested youths. J Behav Med. 2009 Apr;32(2):129–41. doi: 10.1007/s10865-008-9183-2. PMID: 18979194.
- Chapman SL, Wu LT. Substance Apply among Adolescent Mothers: A Review. Child Youth Serv Rev. 2013 May 01;35(5):806-15. doi: 10.1016/j.childyouth.2013.02.004. PMID: 23641120.
- Racz SJ, Saha S, Trent M, et al. Polysubstance Use among Minority Adolescent Males Incarcerated for Serious Offenses. Kid Youth Intendance Forum. 2015 Apr sixteen;45(two):205–20. doi: 10.1007/s10566-015-9334-x. PMID: 26997851.
- Crosnoe R. The Connection Between Academic Failure and Adolescent Drinking in Secondary School. Sociol Educ. 2006;79(1):44–sixty. PMID: 20216913.
- Volkow N. Comorbidity: Addiction and Other Mental Illnesses. National Establish on Drug Abuse. Accessed on 21 January, 2018.
- The National Centre on Addiction and Substance Abuse (CASA). Boyish Substance Use: America's #1 Public Wellness Trouble. Accessed on 22 January, 2018.
- Keyes KM, Brady JE, Li G. Effects of minimum legal drinking age on alcohol and marijuana employ: bear witness from toxicological testing information for fatally injured drivers aged sixteen to 25 years. Inj Epidemiol. 2015 January;2. doi: 10.1186/s40621-014-0032-1. PMID: 26301177.
- Wong SS, Zhou B, Goebert D, et al. The risk of boyish suicide across patterns of drug use: a nationally representative report of high schoolhouse students in the The states from 1999 to 2009. Soc Psychiatry Psychiatr Epidemiol. 2013 Oct;48(10):1611-20. doi: 10.1007/s00127-013-0721-z. PMID: 23744443.
- Johnston LD, O'Malley PM, Miech RA, et al. Monitoring the Time to come national survey results on drug utilize, 1975-2015: Overview, key findings on adolescent drug use. Ann Arbor: Constitute for Social Enquiry, The University of Michigan; 2016. Accessed on October 2, 2018.
- Centre for Behavioral Wellness Statistics and Quality (2016). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA xvi-4984, NSDUH Series H-51).
- Cerda M, Santaella J, Marshall BD, et al. Nonmedical Prescription Opioid Employ in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. J Pediatr. 2015 Sep;167(3):605-12 e1-2. doi: 10.1016/j.jpeds.2015.04.071. PMID: 26054942.
- Curtin SC, Tejada-Vera B, Warner M. Drug Overdose Deaths amongst Adolescents Aged 15-xix in the United states: 1999-2015. NCHS Data Brief. Number 282. National Center for Health Statistics. 2017.
- Bukstein OG, Bernet W, Arnold V, et al. Practice parameter for the assessment and handling of children and adolescents with substance use disorders. J. Am. Acad. Kid Adolesc. Psychiatry. 2005 2005/6;44(6):609-21. doi: 10.1097/01.chi.0000159135.33706.37.
- Hogue A, Henderson CE, Becker SJ, et al. Evidence base on outpatient behavioral treatments for adolescent substance use: Updates and recommendations 2014–2017. Journal of Clinical Child and Adolescent Psychology. in press.
- Hogue A, Henderson CE, Ozechowski TJ, et al. Testify base on outpatient behavioral treatments for adolescent substance utilize: updates and recommendations 2007–2013. J. Clin. Child Adolesc. Psychol. 2014 2014/6/13;43(v):695-720. doi: x.1080/15374416.2014.915550.
- Tanner-Smith EE, Wilson SJ, Lipsey MW. The comparative effectiveness of outpatient treatment for adolescent substance abuse: a meta-assay. J Subst Abuse Treat. 2013 Feb;44(2):145-58. doi: 10.1016/j.jsat.2012.05.006. PMID: 22763198.
- Minozzi S, Amato L, Bellisario C, et al. Maintenance treatments for opiate -dependent adolescents. Cochrane Database Syst Rev. 2014 Jun 24(6):CD007210. doi: 10.1002/14651858.CD007210.pub3. PMID: 24957634.
- Barnett E, Sussman S, Smith C, et al. Motivational Interviewing for boyish substance use: a review of the literature. Addict Behav. 2012 Dec;37(12):1325–34. doi: 10.1016/j.addbeh.2012.07.001. PMID: 22958865.
- Mitchell SG, Gryczynski J, O'Grady KE, et al. SBIRT for adolescent drug and booze utilise: current status and future directions. J Subst Abuse Care for. 2013 May–Jun;44(v):463-72. doi: x.1016/j.jsat.2012.11.005. PMID: 23352110.
- National Constitute on Drug Corruption. Principles of Adolescent Substance Use Disorder Treatment: A Research-Based Guide. January 2014.
- Commission On Substance USE, Prevention. Medication-Assisted Handling of Adolescents With Opioid Use Disorders. Pediatrics. 2016 2016/9;138(3). doi: 10.1542/peds.2016-1893.
- MacIntyre J, Pruitt D, Houston Thousand, et al. Back to Projection Hereafter: plan for the coming decade. Washington, DC: American Academy of Child and Boyish Psychiatry. 2014.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration'southward tool for assessing adventure of bias in randomised trials. BMJ. 2011 Oct 18;343:d5928. doi: x.1136/bmj.d5928. PMID: 22008217.
- Wells GA, Shea B, O'Connell B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Shea BJ, Reeves BC, Wells M, et al. AMSTAR ii: a disquisitional appraisal tool for systematic reviews that include randomised or not-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: ten.1136/bmj.j4008. PMID: 28935701.
- Berkman ND, Lohr KN, Ansari K, et al. AHRQ Methods for Effective Health Care Grading the Strength of a Trunk of Bear witness When Assessing Health Care Interventions for the Constructive Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (Usa); 2008.
- Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a torso of show when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015 Nov;68(11):1312-24. doi: 10.1016/j.jclinepi.2014.11.023. PMID: 25721570.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924–half-dozen. doi: x.1136/bmj.39489.470347.Advertisement. PMID: 18436948.
- Leichsenring F, Abbass A, Driessen Eastward, et al. Equivalence and not-inferiority testing in psychotherapy research. Psychol Med. 2018 Aug;48(eleven):1917–9. doi: 10.1017/S0033291718001289. PMID: 29747714.
VII. Definition of Terms
Cognitive behavioral therapy: a therapy arroyo that aims to modify cognitive processes, beliefs, private behaviors, or environmental reinforcers associated with the adolescent'south substance employ. Variants of this approach include cognitive therapy, dialectical behavior therapy, and the boyish community reinforcement approach.
Contingency direction/motivational incentives: a handling approach that provides the adolescent with tangible rewards for reaching pre-specified treatment goals (east.g., abstinence, attendance, reduced utilise).
Ecological family therapy: a family unit-focused therapy arroyo that expands the boundaries of handling across the family and utilizes individualized strategies to target adolescent substance use in the context of multiple interrelated, nested systems. Case models include multisystemic therapy, multidimensional family unit therapy, family back up network, and ecological based family therapy.
Family unit behavioral therapy: a family unit-focused therapy arroyo that applies principles of operant and social learning inside the family unit context to promote prosocial behaviors and reduce substance utilize.
Family teaching: a family-focused treatment arroyo that focuses on providing didactics most the signs and harms of substance use to the family unit of the adolescent substance user.
Family systems therapy: a family unit-focused therapy approach that attempts to restructure problematic family interaction patterns associated with the adolescent's substance use
Functional family therapy: a family-focused therapy approach that integrates principles of both systems and behavioral approaches.
Integrated handling: a handling arroyo that combines an intervention for substance use and an intervention for a co-occurring mental wellness disorder.
Motivational interviewing/motivational enhancement therapy: a therapy arroyo that focuses on building the adolescent's motivation to reduce his/her substance use.
Multi-component treatment: a treatment arroyo that combines two or more distinct intervention models. Example multi-component approaches include (but are not limited to) motivational enhancement therapy/cognitive behavioral therapy and functional family unit therapy + cerebral behavioral therapy.
Problematic (substance) use: Use of a substance with a negative touch on.
Psychoeducation: a handling approach that aims to provide teaching about the signs and harms of adolescent substance use
Substance employ disorder (SUD): Maladaptive use of a controlled, illicit, or other substance. Per the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-v), An SUD diagnosis is made if an individual exhibits at to the lowest degree two of 11 maladaptive behaviors and symptoms across iv domains (social problems, loss of control, risk behaviors, and physiological changes) within a 12-month menses. Severity may be mild, moderate, or severe.
VIII. Summary of Protocol Amendments
No protocol amendments to engagement.
9. Review of Key Questions
The Bureau for Healthcare Research and Quality (AHRQ) is posting the KQs and protocol on the AHRQ Effective Health Intendance Website for public annotate. The Evidence-based Practice Centre (EPC) will refine and finalize the KQs subsequently review of the public comments, and additional input from Key Informants. The protocol will be further refined based on input from the Technical Skilful Panel (TEP). This input is intended to ensure that the KQs are specific and relevant.
X. Key Informants
Key Informants are the stop users of inquiry, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making health care decisions. Within the EPC plan, the Cardinal Informant role is to provide input into identifying the Key Questions for research that volition inform healthcare decisions. The EPC solicits input from Key Informants when developing questions for systematic review or when identifying high priority research gaps and needed new research. Key Informants are not involved in analyzing the testify or writing the report and have not reviewed the study, except every bit given the opportunity to do so through the peer or public review mechanism.
Fundamental Informants must disclose any financial conflicts of involvement greater than $5,000 and any other relevant business or professional conflicts of interest. Because of their role as end-users, individuals are invited to serve as Fundamental Informants and those who present with potential conflicts may be retained. The AHRQ Task Order Officer (TOO) and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
XI. Technical Experts
Technical Experts found a multi-disciplinary group of clinical, content, and methodological experts who provide input in defining populations, interventions, comparisons, or outcomes and identify particular studies or databases to search. They are selected to provide wide expertise and perspectives specific to the topic nether development. Divergent and alien opinions are common and perceived equally healthy scientific discourse that results in a thoughtful, relevant systematic review. Therefore, study questions, design, and methodological approaches do not necessarily stand for the views of individual technical and content experts. Technical Experts provide data to the EPC to identify literature search strategies and suggest approaches to specific issues as requested past the EPC. Technical Experts exercise not practice analysis of whatever kind nor practise they contribute to the writing of the study. They have not reviewed the report, except every bit given the opportunity to do so through the peer or public review mechanism.
Technical Experts must disclose any fiscal conflicts of interest greater than $five,000 and any other relevant business organization or professional conflicts of interest. Considering of their unique clinical or content expertise, individuals are invited to serve as Technical Experts and those who present with potential conflicts may be retained. The AHRQ Also and the EPC work to residuum, manage, or mitigate whatever potential conflicts of involvement identified.
XII. Peer Reviewers
Peer reviewers are invited to provide written comments on the draft report based on their clinical, content, or methodological expertise. The EPC considers all peer review comments on the typhoon report in training of the terminal report. Peer reviewers do non participate in writing or editing of the final report or other products. The final study does non necessarily stand for the views of individual reviewers. The EPC will consummate a disposition of all peer review comments. The disposition of comments for systematic reviews and technical briefs will be published three months after the publication of the testify report.
Potential Peer Reviewers must disembalm any financial conflicts of involvement greater than $v,000 and any other relevant business or professional conflicts of involvement. Invited Peer Reviewers may not have any financial conflict of interest greater than $v,000. Peer reviewers who disclose potential business or professional conflicts of interest may submit comments on typhoon reports through the public annotate machinery.
Xiii. EPC Squad Disclosures
EPC core team members must disclose any financial conflicts of interest greater than $1,000 and any other relevant business or professional person conflicts of interest. Related fiscal conflicts of interest that cumulatively total greater than $one,000 will usually disqualify EPC core team investigators.
14. Role of the Funder
This project was funded under Contract No. HHSA 290-2015-00002-I from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The AHRQ Chore Order Officeholder reviewed contract deliverables for adherence to contract requirements and quality. The authors of this report are responsible for its content. Statements in the report should non be construed as endorsement by the Agency for Healthcare Research and Quality or the U.Southward. Department of Health and Human Services.
XV. Registration
This protocol will be registered in the international prospective annals of systematic reviews (PROSPERO).
[1] Substances considered: alcohol, cannabis, opioids, sedatives/hypnotics/anxiolytics, stimulants, inhalants and hallucinogens. Tobacco is excluded.
[2] Subpopulations considered: psychiatric co-morbidities, age (early, center and late boyhood), sex and gender, race/ethnicity, socioeconomic status and related characteristics (east.g., homelessness, poverty), meaning, postpartum, and parenting adolescents, demographic/family characteristics. Factors in bold will be prioritized if necessary.
[iii] Intervention characteristics: target (e.thousand. teen, family or group of teens), duration and setting.
Source: https://effectivehealthcare.ahrq.gov/products/substance-use-disorders-adolescents/protocol
0 Response to "Substance Abuse in Children Ages 11-13 Peer Reviewed"
Post a Comment